- Time:2022.11.18
- Browse:1100

The 86th
China International Medical Equipment Fair (CMEF)
will be held in
Shenzhen International Exhibition and Convention Center (Bao'an)
from November 23rd-26th!
From November 23rd to 26th, 2022, the 86th CMEF China International Medical Equipment Fair, the global industry wind vane "carrier level" medical industry event, will be launched in Shenzhen World Exhibition and Convention Center (Bao'an District).
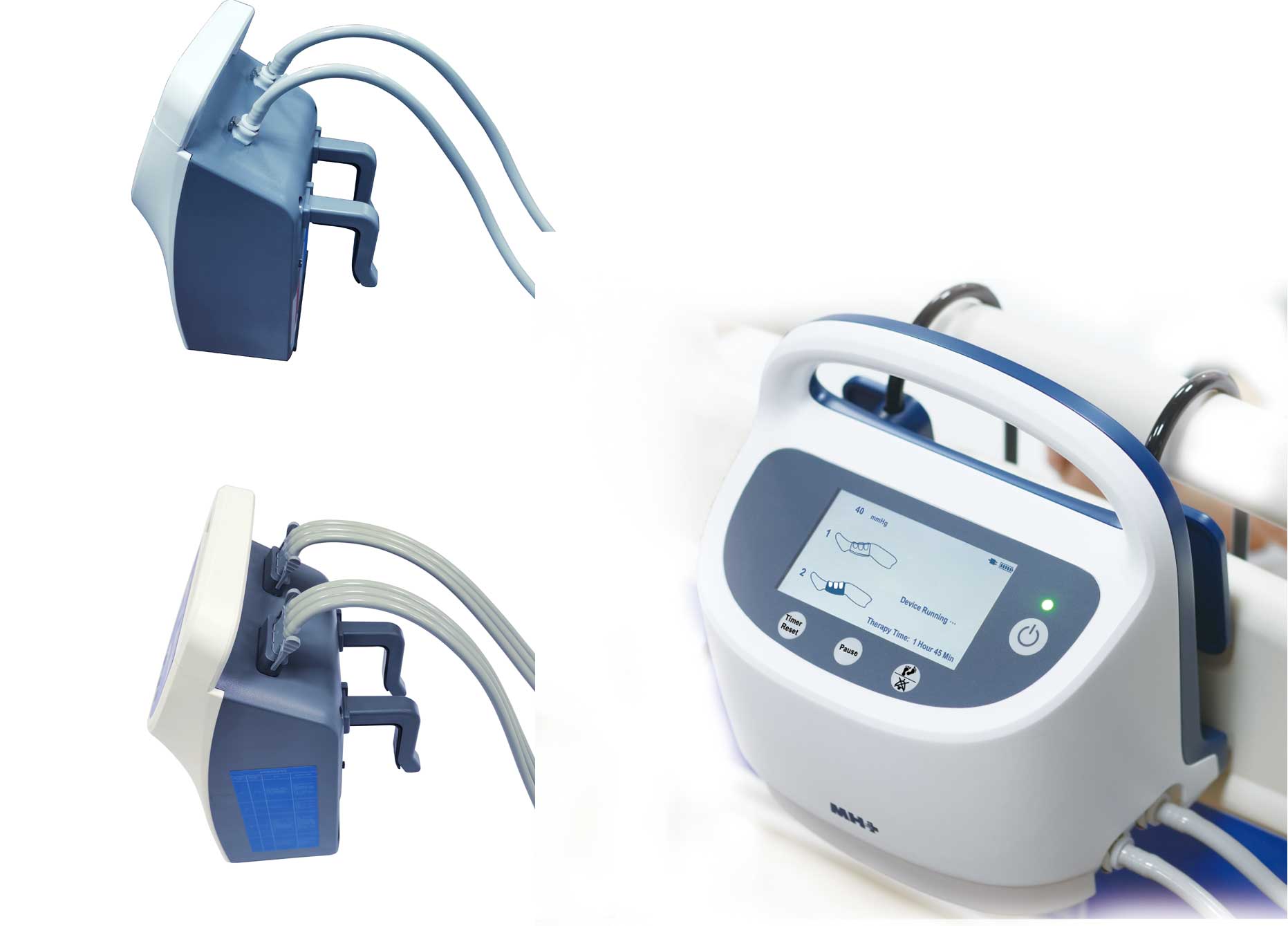
At that time, as a manufacturer of deep vein thrombosis prevention system with more than 11 years of experience and a partner of the world's top 500 enterprises, MedHealth Medical will bring core equipment and products such as deep vein thrombosis pump, limb pressure sleeve and a new generation of DVT pump to booth 12T56 in Hall 12! We sincerely invite you to join us, and welcome you to visit us!
Exibition Information
Show Schedule
| Event | Notes | Date | Time |
|
Stand Build-up (raw space) |
Hall 9/10/11/12/14/20 |
Nov 20th to Nov 22nd |
09:00-17:00 |
|
Hall 13/15/16/17 |
Nov 19th to Nov 22nd |
09:00-17:00 |
|
|
Stand Set-up (all space) |
All halls |
Nov 21st to Nov 22nd |
08:30-18:00 |
|
Exhibitor Registration |
North Registration Hall |
Nov 21st |
09:00-18:00 |
|
Nov 22nd |
08:30-18:00 |
||
|
Show Opening Hours |
Exhibitors |
Nov 23rd to Nov 25th |
08:30-17:00 |
|
Nov 26th |
08:30-16:0 |
||
|
Visitors |
Nov 23rd to Nov 25th |
09:00-17:00 |
|
|
Nov 26th |
09:00-16:00 |
||
|
Removal of Exhibits & Dismantling of Stands |
All halls |
Nov 26th 17:00-22:00 |
|
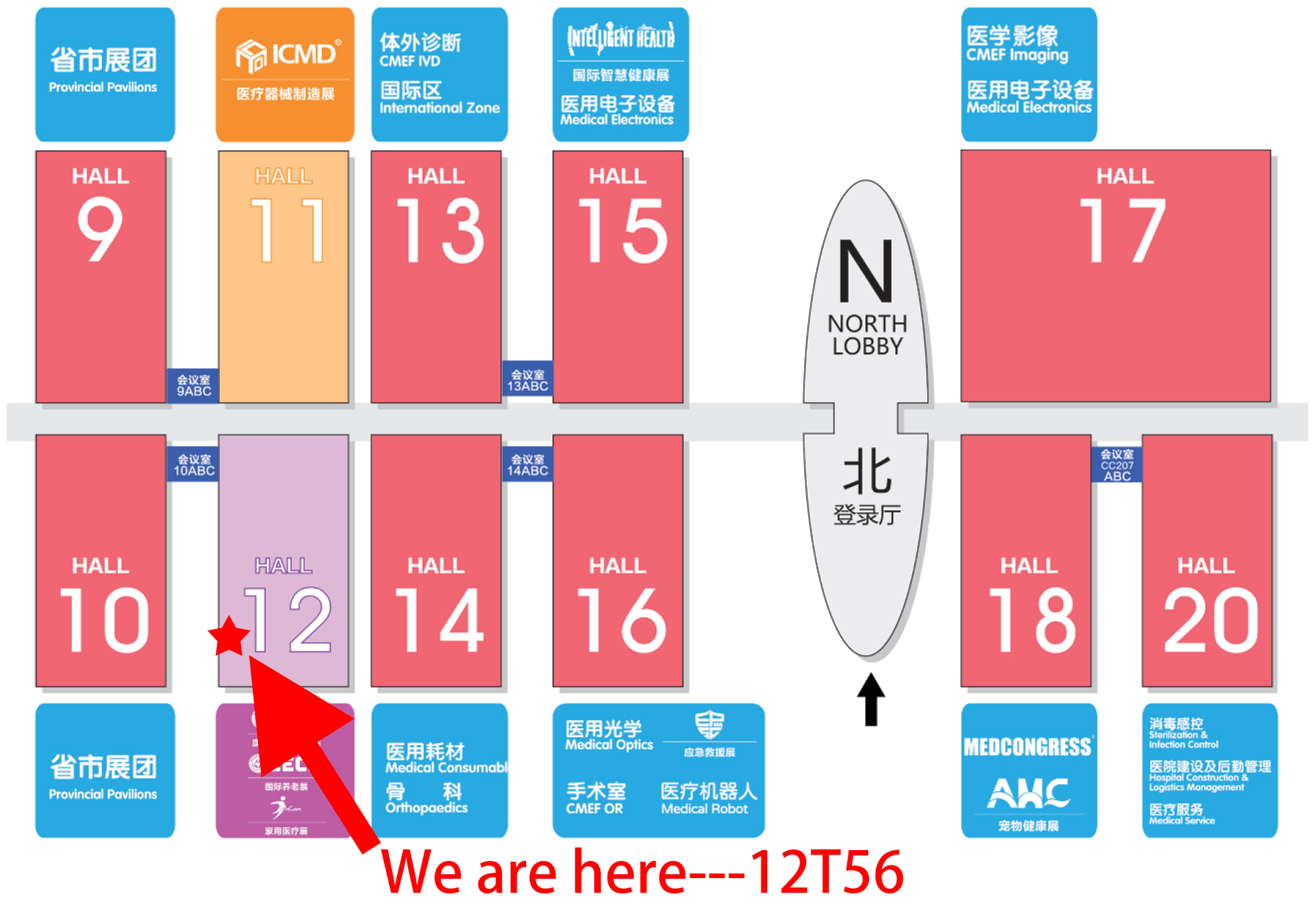
Address
MedHealth Medical Booth
12T56


Notes
* Please register with your name and mobile phone number consistent with your ID card, and bring your ID card to the site.
* The white list review results are obtained from the big data screening of epidemic prevention related departments. Please register as soon as possible. The white list review results will be sent to you by SMS, and you can enter the site only after the results are passed!
Our Products
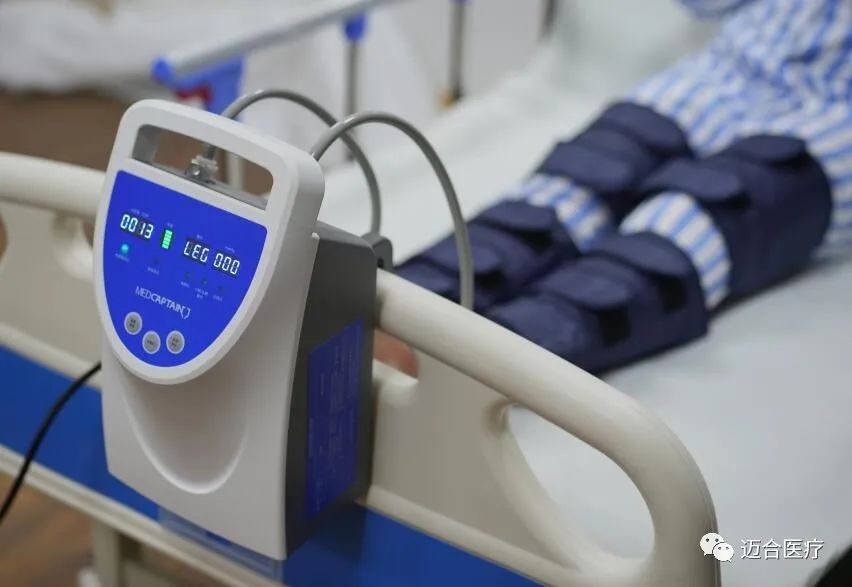
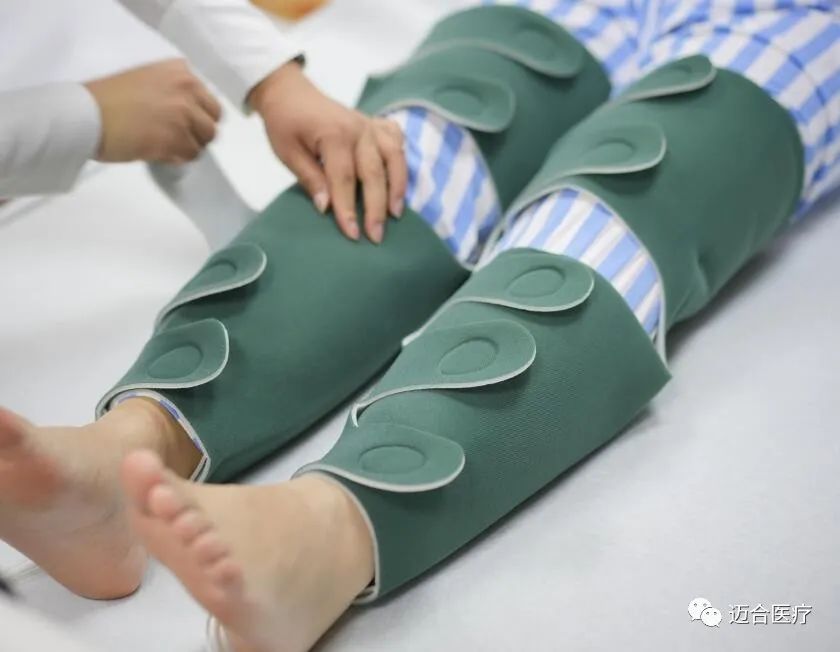
MedHealth Medical Limited strictly complies with ISO13485, 21CFR820 and GMP quality systems to ensure stable and reliable product quality. It passed the FDA inspection in 2015 with zero defect (NAI), and passed the ISO13485 quality management system audit in 2016. In 2020, it passed the TUV SUD review and conforms to the EN ISO13485:2016 quality system. In the same year, it passed the review of the State Food and Drug Administration audit, obtained the production license for Class II medical products, and began to carry out domestic medical device business. The products have been exported to more than 40 countries.
To know more about MedHealth Products
Welcome to visit our booth: Hall 12-T56
November 23-26, 2022
Shenzhen World Exhibition & Convention Center (Bao'an)
Looking forward to meeting you!